[Nano Lett.] Macroscopic Salt Rejection through Electrostatically Gated Porous Graphene
We domonstrate a new physical mechanism that rejects salt diffusion through nanoporous graphene even when the pore size is significantly larger than the hydrated radii.
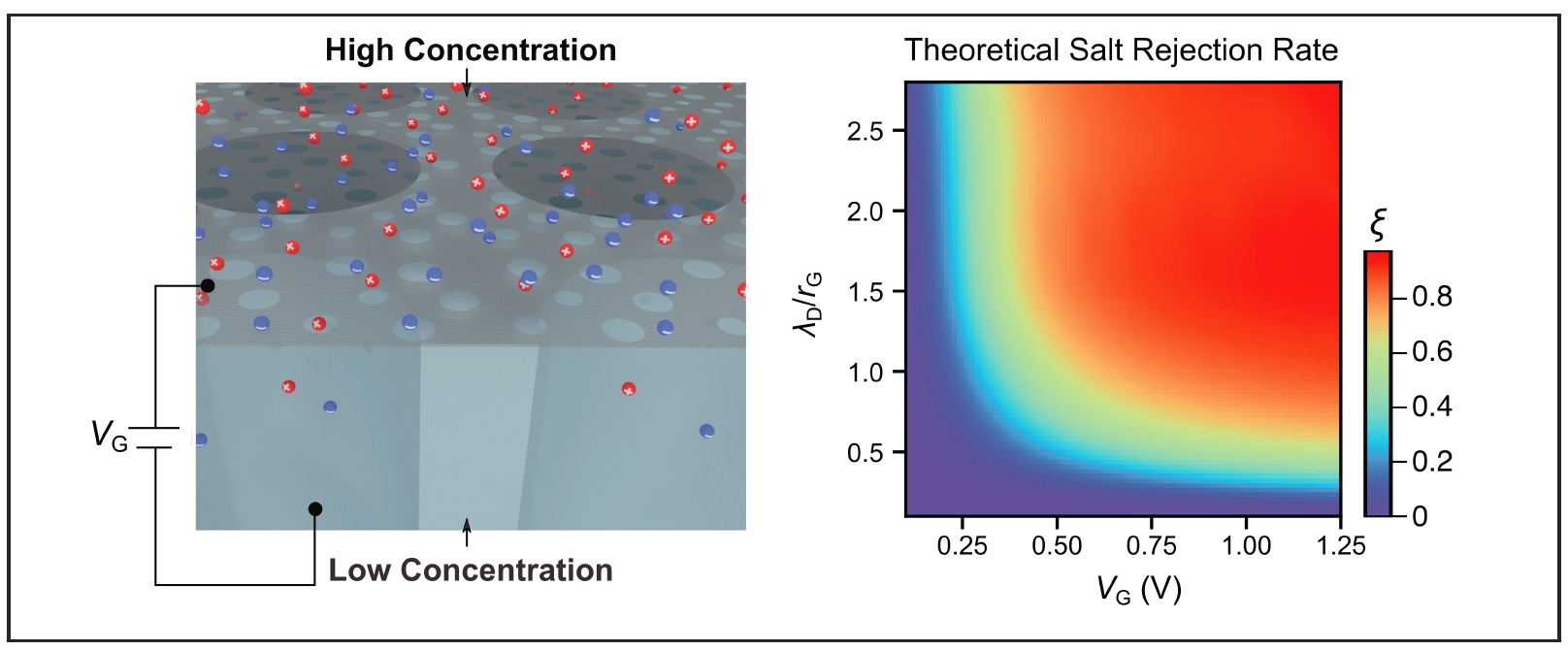
Atomically thin porous graphene is emerging as one of the most promising candidates for next-generation membrane material owing to the ultrahigh permeation. However, the transport selectivity relies on the precise control over pore size and shape which considerably compromises the scalability. Here, we study electrolyte permeation through a sheet of large-area, porous graphene, with relatively large pore sizes of 20±10 nanometers. Counterintuitively, a high degree of salt rejection is observed by electrostatic gating, reducing the diffusive flux by up to one order of magnitude. We systematically investigate the effects of salt concentration and species, including developing a theory to model the electrolyte diffusion through a nanopore drilled in a sheet of gated graphene. The interplay between graphene quantum capacitance and the electrical double layer is found to selectively modulate the anionic and cationic transport paths, creating voltage-dependent electrochemical barriers when the pore size is comparable to the Debye length. Our findings reveal a new degree of freedom regulating electrolyte permeation through porous two-dimensional materials, complementary to the pore size design and engineering.
